USA partially stopping using Hydroxychloroquine for Covid-19
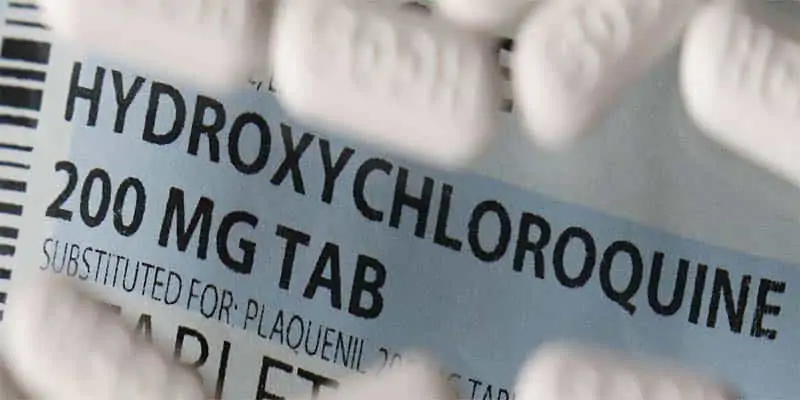
The US is partly stopping using hydroxychloroquine (HCQ), which President Donald Trump personally acquired from India, and chloroquine (CQ) to take care of COVID-19, suspending it in hospital settings but enabling it everywhere under physician’s care.
However, Health Secretary Alex Azar explained the FDA ruling was restricted to hospital usage together with “the most extreme instances” and the medications have never been fully banned and physicians can continue to prescribe them.
“We continue to examine in out-patient configurations, in addition to preventative.
When Trump was asked about the FDA decision by colleagues in the White House, he deferred to Azar, who stated:”If a physician wants to prescribe it, working with a patient, then they might prescribe it for any function they want to do so.
Also Read
“They could possibly be utilized in hospitals, so they could possibly be utilized in outpatient; they might be used in the home, all subject to some physician’s prescription.”
The FDA reported the withdrawal of the EUA has been “in light of continuing severe cardiac adverse events as well as other possible serious side effects, the known and potential advantages of both chloroquine and hydroxychloroquine no longer outweigh the known and possible dangers”.
The EUA permits medication to treat a disease on a crisis basis without a authorization for the disorder for.
Even the restriction on HCQ usage is a success for the Democrats that were opposing it afterwards trump had taken it as a preventative for COVID-19 to 14 days and had thrown his weight.
Even the FDA’s assertion that info hadn’t proven that HCQ and CQ were powerful is at odds with a different advancement that dealt a blow to the credibility of the esteemed medical journal, The Lancet within a study that asserted HCQ wasn’t powerful and seemed to raise the risk of passing due to doubts regarding the information utilized.
Also Read
The Lancet needed to “deeply apologies” and draw the much-cited research when experts challenged the trustworthiness of the information utilized in the analysis by three Indian source scientists and yet another.
The information came from a firm named Surgisphere, who’s CEO Sapan Desai was recorded as author of this analysis.
After it had been removed, the World Health Organization who had ceased HCQ trials dependent on the Lancet study resumed them.
Azar explained although employed to CQ produced but didn’t mention that the HCQ.





