What is Smog? How it is formed? Discuss its effects.
Smog is a mixture of smoke and fog. The name smog is derived from combination. of smoke and fog. “Smoke + Fog → Smog”, Smog is of two types: classical smog and photo-chemical smog.
Classical Smog:
It is commonly know as smog and is produced from gases like SO2, and NO present in air (produced by burning of fossil fuels like coal and petrol or gasoline in homes, industries and automobiles). These gases get mixed with moisture in the tiny droplets of fog producing H2SO4 and HNO3, respectively.
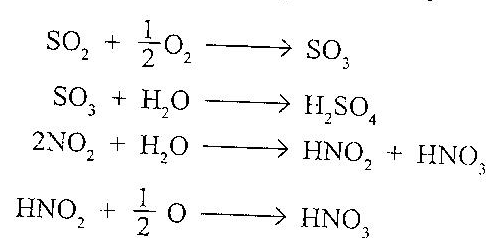
The resulting mixture consisting of H2SO4 and HNO, get condensed on the solid particulate matter of smoke present in the air to give a colloidal dispersion called smog or classical smog.
Usually smog is formed during winter season and is present in the lower atmosphere. It decreases visibility and affects human health. It causes breathing problems like bronchitis and asthma, which may be responsible for heart problems It produces irritation to eyes, nose and throat. Due to reduced visibility there are problems in road and rail traffic.
It is well known that in December 1952, a dense cloud it smog was formed over London city and remained for 5 days. It resulted in the death of about 5000 people and created health problems to numerous other people. The cause of death was pneumonia, bronchitis and other respiratory problems. This smog commonly called London smog formed after the introduction of coal as a fuel which produced both SO2 and smoke. The London smog is also called Reducing Smog.
Photo-Chemical Smog:
This type of Smog is formed by the combination of smoke, dust and fog with air pollutants particularly oxides of nitrogen and hydrocarbons. These pollutants originate from automobile exhaust and other industrial activities. The formation of photo-chemical smog can be explained in the following way (image below). The various steps involved are:
The hydrocarbons (which are reactive containing a C=C group) from automobiles interact with ozone to form a hydrocarbon free radical (RCH2) (1). These free radicals may also be formed by the reaction of hydrocarbons with atomic oxygen [evolved from NO2, by uv radiation from the sun; NO2, + uv radiation > NO + O]
The hydrocarbon free radicals (1) react rapidly with oxygen to form another free radical RCH2O2(2).
The second free radical (2), in turn, reacts with nitric oxide present in the atmosphere (either from N and O in presence of sun light or by irradiation of NO2) to produce the third free radical, RCH2O (3) (the NO is converted into NO2).
The third free radical, R CH2O’ (3) subsequently reacts with oxygen yielding an aldehyde, RCHO and generating a hydroperoxyl radical (H2O).
The hydroperoxyl radical (HO2,) reacts with nitric oxide to give NO2, and generating hydroxyl radical (HO).
The hydroxyl radical (HO) being extremely reactive reacts with a hydrocarbon, regenerating the hydrocarbon free radical, RCH2 (1).
The above cycle is repeated a number of times leading to the rapid build up of photo-chemical smog products.
The aldehyde (RCHO) formed in step (iv) reacts with hydroxyl radical (HO) producing an acyl radical (RCO), which in turn reacts with oxygen to give peroxyacyl radical (RCOO2) The formed peroxyacetyl radical finally reacts with nitrogen dioxide (NO2) to generate peroxyacetylnitrate (PAN). The steps involve in the last sequence of reactions from the aldehycle are shown in Figure below.

It is the peroxyacetylnitrate (PAN), a constituent of smog, which produces irritation to eyes. The two components of smog, viz, ozone and PAN affect the respiratory tract of human beings. It also causes nose and throat irritation and can lead to several problems of eyes, lungs and heart. The photo-chemical smog also affects the growth of plants and cause damage to the vegetation.
The formation of photo-chemical smog can be suppressed by preventing the release of oxides of nitrogen and hydrocarbons in the atmosphere by motar vehicles by using catalytic converters.
The same objective can be achieved by using a better quality of oil, so that there is no emission of the oxides of nitrogen and hydrocarbons. This is possible by the use of so called Reformed gasoline, in which about 10% of the aromatic hydrocarbons are replaced by fuel oxygenates. such as methyl tertiarybutyl either.
The additives make the fuel burn more efficiently. With this, the emission of volatile organic hydrocarbons is suppressed. Also, the gasoline containing oxygenates burns at a lower temperature resulting in the reduction of the emission of the oxides of nitrogen. Also, with the advancement of technological developments, it is now possible to fabricate automobile engines so that there is no urburnt hydrocarbons left. This technology has been used in most of the advanced countries.


